- Valtria in News -
Application of GMP regulations in medical cannabis
1. Introduction
2. Medicinal Cannabis
Cannabis is a special case within standard medicine manufacturing practices for the following reasons:
- While many plant-based medicines or APIs and supplements exist, they reach the end consumer/patient in the form of extracts.
- No other plant, such as cannabis, can be found in the original form of a flower, apart from the conventional formats. (Herbal formulations can be left aside, as they have gradually disappeared from the pharmacopoeia and do not fall under the definition of medicines with therapeutic effect)
- Cannabis is a very potent API, with multiple combinations of components (cannabinoids, terpenes) and multiple therapeutic applications, some of them with side effects or psychotropic effects (with associated safety implications).
- The main characteristics of cannabis as an API are given by genetics, but during the growth of the plant, it can greatly change its potency (concentration).
- Cannabis has opened the door to a new professional profile outside the pharma production sector, which has led to discrepancies in the application of GMP regulations in this process.
3. Application of GMP regulations
Let’s look at some particularities of the application of these GMP regulations in Cannabis production, based on our experience and our product and process risk analyses for cannabis.
Dried flower of Cannabis "direct use" as a medicinal product.
According to table 1 of the application of the GMP regulations to the manufacture of active substances of plant origin, we would be placed in the third row:
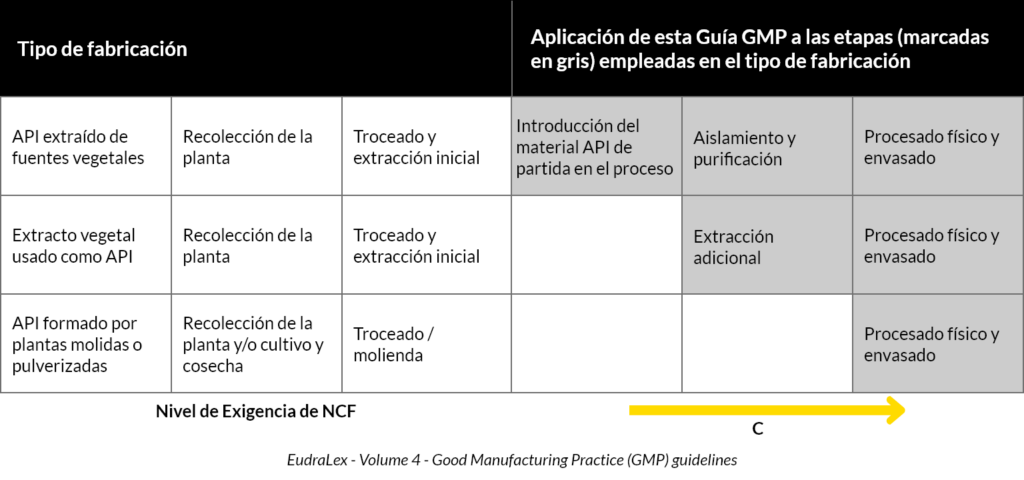
Following the indications in the table, GMP regulations will start to be applied at the time of physical processing and packaging including drying and shaving (last processing steps before dispensing to patients).
As GMP regulations apply only to the final processing steps, the application of GACP regulations during the cultivation steps is of particular importance due to the following aspects:
- The different active ingredients are created in an uncontrolled environment (according to Pharma criteria) for more than 10 weeks, this is an atypical case. If we draw the parallel with the formulation of an API, GMPs apply at the time when the active molecule appears. Not here, the active molecule appears and is formed long before we enter the GMP requirement.
- Environmental conditions (temperature, humidity and light) as well as cultivation processes and strategies (density, irrigation, fertilisers, …) greatly influence the composition of the different APIs and the yield of the crop.
- Any of the above parameters out of control may invalidate the product completely. Knowing that GMPs are not mandatory, the application of GACP becomes even more important.
- Evaluation of suppliers, training of personnel, condition of facilities require very rigorous procedures, even if they are not GMP.
It is essential to control the different parameters that make up the cultivation phase in order to ensure the quality, stability and yield of the crop.
At the same time, keeping the above parameters under control means higher investment and operating costs, so it is necessary to find a balance.
Dried flower of Cannabis "direct use" as a medicine
When it comes to defining the classification of the controlled environment for processing cannabis, we return to GMP and are left without a clear answer. In Annex 7, it simply tells us to implement particular measures to avoid cross-contamination.
From Chapter 1 of the new Annex 1 and its “principles and guidance, such as contamination control strategy, facility design, clean room classification, qualification, validation, monitoring and personnel clothing, can be used to support the manufacture of other products that are not intended to be sterile, such as certain liquids, creams, ointments and biological intermediates with low microbial load, but where the control and reduction of microbial contamination is considered important”.
We are left with the choice of going to ISO 8, as a minimum precaution to respond to the characteristics of this product. But why not another classification?
Let’s take a look at some particularities:
- This is a “mono-product” production, but due to some long process times (drying, curing) different batches can overlap in the production space.
- Unlike other pharma products, the risk of cross-contamination does not arise from mixtures of active ingredients, even if different genetics are processed.
- The greatest risk is biological (fungi, bacteria…), either from the product itself or from external agents. This biological contamination may be evident and detectable in the early GMP and quality control phases of the batch, or it may remain latent and develop in the first days of the drying phase, where environmental conditions are still suitable for the proliferation of fungi and bacteria.
- According to our analysis, the greatest risk of cross-contamination would be in the wet processing room and in the drying room. For these rooms we advise a design of containment pressures and rigorous procedures regarding PPE and corrective actions in case of contamination detection.
- It is important to note that during the process the product is exposed for a long time (even days) to the environment of the room that acts as a container. Therefore, room cleaning and disinfection procedures must be thorough. Here we are going back to Annex 1, with the obligation to develop a fine-tuned CCS, “Contamination Control Strategy”, adapted to the peculiarity that most of the time, the room, the environment, with its operators, is assimilated to the container, up to the packaging stage.
Batch definition
In GMP, reference is always made to the manufacturing batch, i.e. a fixed quantity of product obtained under the same conditions and process to ensure uniformity. This implies a great deal of complexity in medical cannabis. Once the application of GACP ensures maximum uniformity, within the possibilities of the cultivation facilities available, the GMP process ensures that this uniformity is maintained:
- The processes are slow (a batch can take between one and 2 work shifts to process), and exposed to an environment to which it is sensitive. This environment has to be suitable and controlled to ensure that the first and the last product processed has the same characteristics.
- In the case of drying, you have a large volume of product that is a “living” material, which evolves over several days. The installations have to be of optimal design, duly validated (temperature and humidity mapping, drying recipes, filling procedures, etc.).
- Many processes require human intervention. Let’s think about different phases for example manual shaving or curing time and conditions where decisions are subjective.
- Finally, the size of each batch, after the long process of more than 100 days from the procurement of cuttings to the final product, can vary greatly. The reconciliation between the number of plants, discards, waste and the final product obtained is very complex and critical in the case of a narcotic drug.
Flower for medical Cannabis extracts
At this point, we would be in the first and second row of table 1 above (application of GMP Guide).
In our experience in the case of a product where only one extraction is performed, this must be done in a GMP environment. On the other hand, if extraction plus distillation or purification takes place, extraction could be performed in a NON-GMP environment, as long as the following steps necessarily take place in a GMP environment.
The more purified the product to be obtained, the more variability the starting material (flower) can have, although we will have an important impact on the yield (costs) of our process. This variability in flower characteristics would allow for a less specialised cultivation infrastructure but does not exempt from strict compliance with GACP standards, as an out-of-parameter plant source does not allow for compliance with GMP process standards.
For full spectrum extractions (first extraction) where the presence of other cannabinoids or terpenes is assessed, the starting point is very specific genetics that are even associated with the description that accompanies the final product. The quality of the starting flower is crucial (it would correspond to the function of a reactor for chemical APIs). For this “GMP flower for extraction” quality, it is necessary that the person responsible for the medicinal product is involved in the definition of the procurement process.
4. Conclusions
We see that shedding light on how to apply GMP regulations in the production of this particular medicine requires detailed knowledge of the product and the process. We have to go and look for part of the answers in the new Annex 1, and force ourselves to develop a CCS, with a scientific basis of risk analysis.
This GMP approach to the production process (certified by the AEMPS), in addition to ensuring the quality and stability of the products, is the basic and mandatory step to consider cannabis as a medicine and will keep it away from negative perceptions by society.
5. Bibliography
- Normas de Correcta Fabricación de Medicamentos: la Directiva 2003/94/CE, (Commission Directive (EU) 2017/1572 for medicinal products for human use- en particular annexo 7, annexo1
- ICHQ9: Quality risk managment_2005
- ICHQ7A: GMP active pharmaceutical ingredients
- ICH Q10 : PHARMACEUTICAL QUALITY SYSTEM
- Pharmacopoea Helvetica 11.3 Ed-fr_2019 : Flor de cannabis, CH211-S-263
- Analytical Monograph Cannabis Flos – OMC / Farmalyse BV V7, Nov 28.2014
- Bundesinstitut für Arzneimittel und Medizinprodukte Bekanntmachung zum Deutschen Arzneibuch 2018* Vom 9. April 2018
- PIC/S GUIDE TO GOOD MANUFACTURING PRACTICE FOR MEDICINAL PRODUCTS
Eudald Bogatell
Project Engineer at VALTRIA
Lidia Campillo y Frederic Pascual
Collaborators